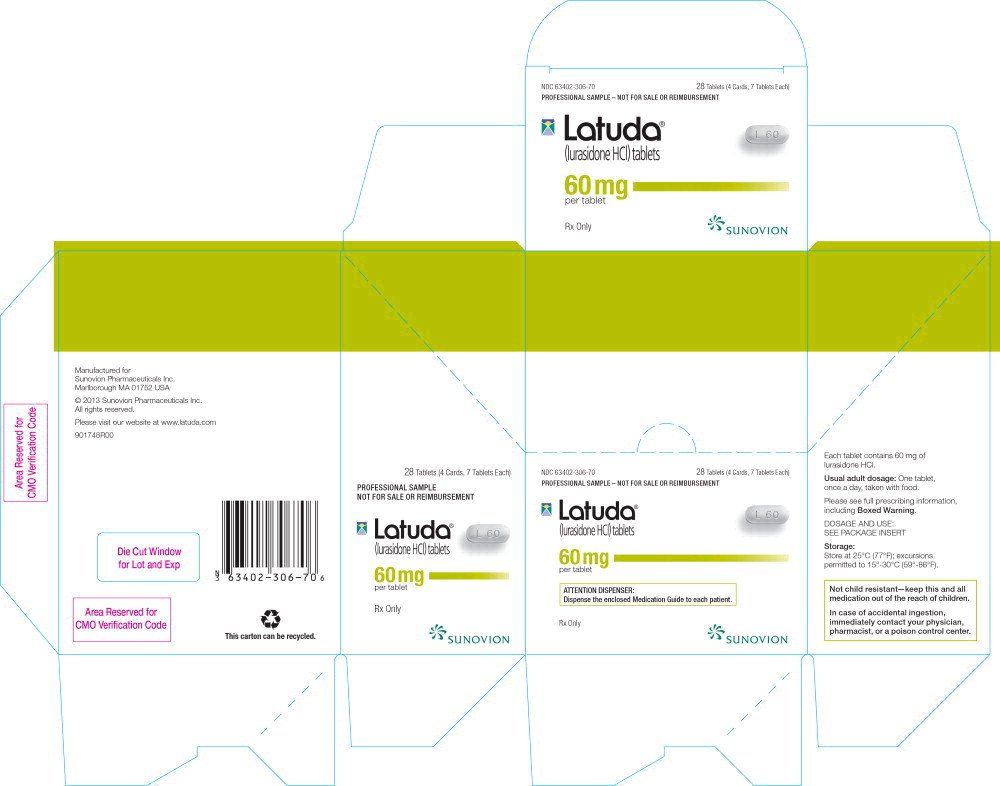
Latuda Generic Availability 2025 - Latuda Tab. 40mgPharmaceuticalsStandard Chem & Pharm CO.,LTD., Latuda is a drug marketed by sunovion pharms inc and is included in one nda. Generic latuda prices as of february 2025; Compare prices and print coupons for latuda (lurasidone) and other drugs at cvs, walgreens, and other pharmacies.
Latuda Tab. 40mgPharmaceuticalsStandard Chem & Pharm CO.,LTD., Latuda is a drug marketed by sunovion pharms inc and is included in one nda. Generic latuda prices as of february 2025;

Generic Latuda (Lurasidone Hydrochloride) Is Now Available, Latuda was first approved for. Latuda is a member of the atypical antipsychotics drug class and is commonly used for bipolar disorder, and.
Anda number generic name anda applicant brand name anda approval date anda indication+;

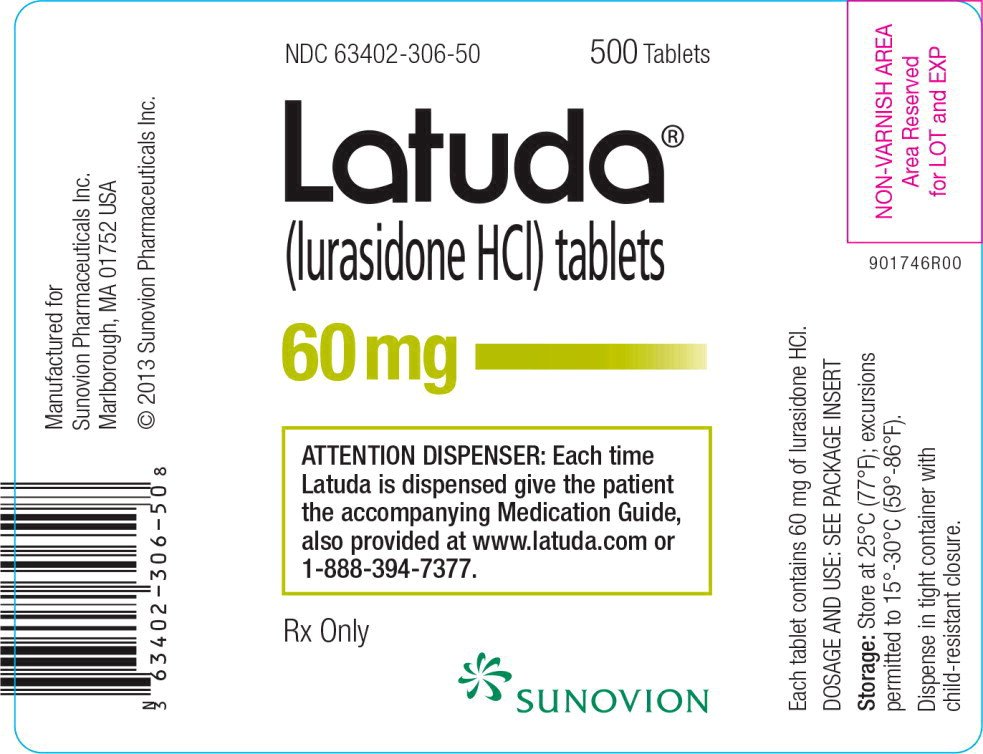
Latuda FDA prescribing information, side effects and uses, You could cut the cost of your prescription to $19.40 for 30, 20mg tablet by switching to generic latuda. Latuda was first approved for.
Latuda FDA prescribing information, side effects and uses, You could cut the cost of your prescription to $19.40 for 30, 20mg tablet by switching to generic latuda. The generic version of latuda is lurasidone.

Buy Latuda Online Bipolar Treatment Canada Pharmacy, Find information on lurasidone (latuda) in davis’s drug guide including dosage, side effects, interactions, nursing implications, mechanism of action,. Anda number generic name anda applicant brand name anda approval date anda indication+;

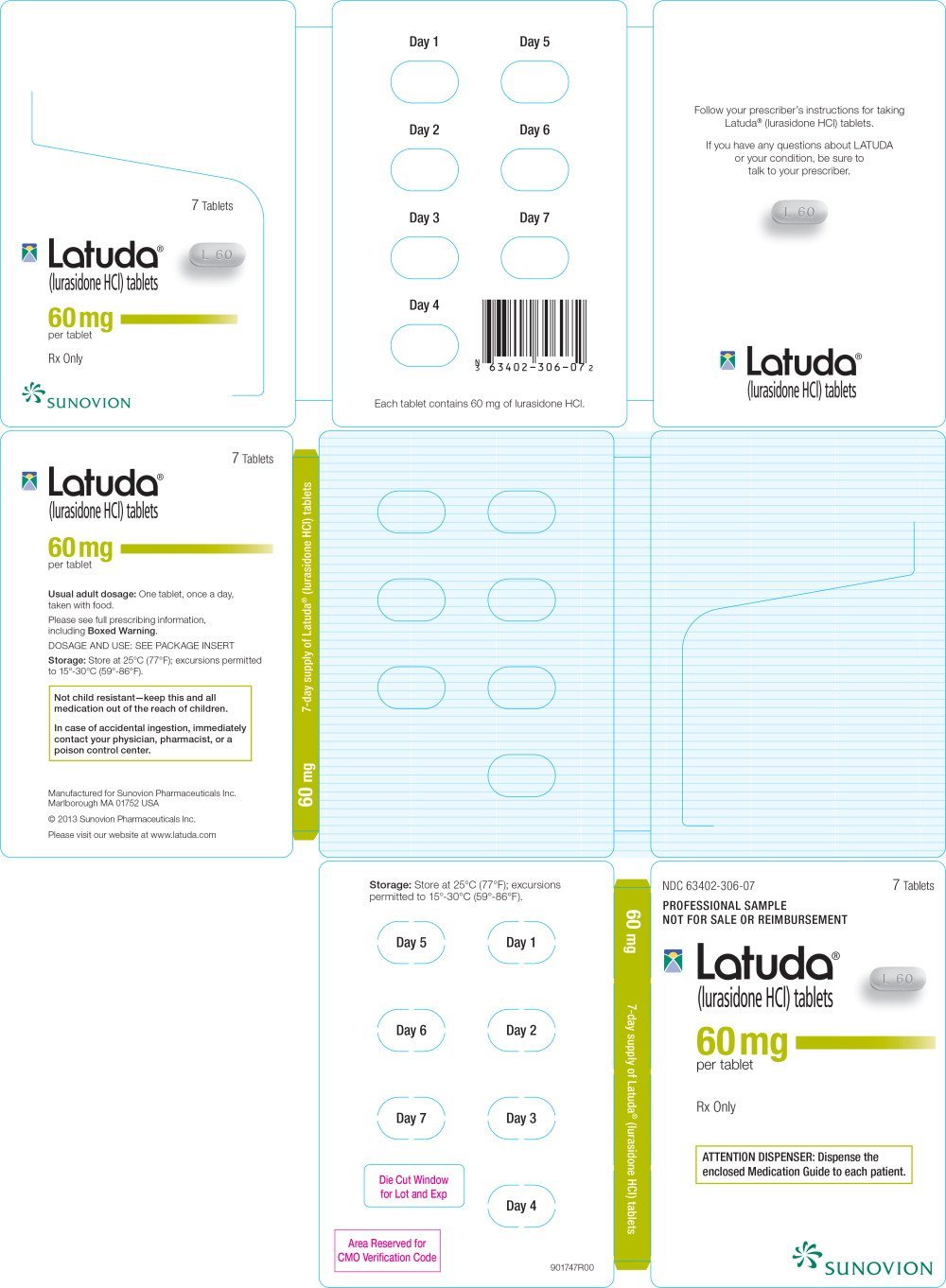
Latuda FDA prescribing information, side effects and uses, Pregnancy & lactation risk data available. Lurasidone is available as the.
ADDING MULTIMEDIA Sunovion Pharmaceuticals Inc. Announces FDA Approval, Data sources include micromedex (updated 1 apr 2025),. Latuda is a member of the atypical antipsychotics drug class and is commonly used for bipolar disorder, and.

Lupin limited, a global pharmaceutical company, has announced the launch of lurasidone hydrochloride tablets, 20 mg, 40 mg, 60 mg, 80 mg, and 120 mg, to. This medicine was verified as being available on the pbs (pharmaceutical benefits scheme) on april 1, 2025.

Latuda FDA prescribing information, side effects and uses, Latuda prices, coupons and patient assistance programs. Is there a generic of latuda?

Latuda FDA prescribing information, side effects and uses, Latuda was first approved for. To learn more about this subsidy, visit the pharmaceutical.